Do flying beetles respond to human-dominated landscapes as complex mosaics or binary patterns
DOI:
https://doi.org/10.3097/LO.201016Keywords:
Cerambycidae, Functional connectivity, Habitat, Landscape mosaic, Least-cost path, Resistance surfaceAbstract
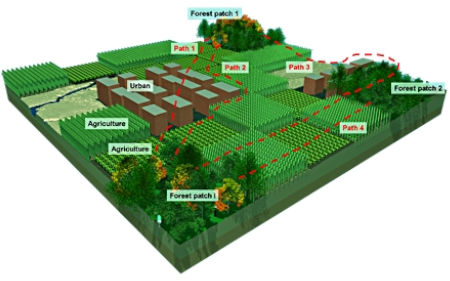
Understanding and measuring functional connectivity for animals with habitats that have been fragmented by human activity requires that the biology and movement of the species be considered. We used least cost paths in GIS to test hypotheses regarding how different species of longhorned beetles likely connect habitats with dispersal. We predicted that there would be differences in the functional connectivity of landscapes depending on species larval niche breadth, adult feeding habits, and the potential for use of non-forest habitats. For the species with very specialized larvae, we developed a classification tree to determine areas likely to contain the appropriate species of host tree. Connectivity calculated using least cost paths did not out-perform Euclidean distances for three generalist beetles. This was also the case for the specialist beetle species when all forest was considered habitat. However, when we delineated habitat based on areas likely to support the host tree the functional connectivity incorporating least cost paths was a much better predictor than that using Euclidean distances. Generalists may respond to fragmented habitat in a binary habitat-matrix way while more specialized species may respond to a mosaic. These trends are obscured if habitat is defined by human perceptions rather than species biology.
References
Adriaensen, F., Chardon, J. P., De Blust, G., Swinnen, E., Villalba, S., Gulinck, H., & Matthysen, E. (2003). The application of 'least-cost' modelling as a functional landscape model. Landscape and Urban Planning, 64(4), 233-247. doi:10.1016/S0169-2046(02)00242-6
Allison, J. D., Borden, J. H., & Seybold, S. J. (2004). A review of the chemical ecology of the cerambycidae (coleoptera). Chemoecology, 14(3-4), 123-150. doi:10.1007/s00049-004-0277-1
Bélisle, M. (2005). Measuring landscape connectivity: The challenge of behavioral landscape ecology. Ecology, 86(8), 1988-1995. doi:10.1890/04-0923
Bender, D. J., & Fahrig, L. (2005). Matrix structure obscures the relationship between interpatch movement and patch size and isolation. Ecology, 86(4), 1023-1033. doi:10.1890/03-0769
Bergin, T. M., Best, L. B., Freemark, K. E., & Koehler, K. J. (2000). Effects of landscape structure on nest predation in roadsides of a midwestern agroecosystem: A multiscale analysis. Landscape Ecology, 15(2), 131-143. doi:10.1023/A:1008112825655
Blackman, M. W. (1918).
Brattli, J. G., Andersen, J., & Nilssen, A. C. (1998). Primary attraction and host tree selection in deciduous and conifer living coleoptera: Scolytidae, curculionidae, cerambycidae and lymexylidae. Journal of Applied Entomology, 122(7), 345-352. doi:10.1111/j.1439-0418.1998.tb01511.x
Chénier, J. V. R., & Philogéne, B. J. R. (1989). Evaluation of three trap designs for the capture of conifer-feeding beetles and other forest coleoptera. The Canadian Entomologist, 121(2), 159-167. doi:10.4039/Ent121159-2
Chénier, J. V. R., & Philogène, B. J. R. (1989). Field responses of certain forest coleoptera to conifer monoterpenes and ethanol. Journal of Chemical Ecology, 15(6), 1729-1745. doi:10.1007/BF01012261
Collinge, S. K., & Palmer, T. M. (2002). The influences of patch shape and boundary contrast on insect response to fragmentation in california grasslands. Landscape Ecology, 17(7), 647-656. doi:10.1023/A:1021536302195
Craighead, F. C. (1950). Insect enemies of eastern forests. Miscellaneous Publications, United States Department of Agriculture, 657, 1-679.
Deam, C. C. (1931). Trees of indiana. fort wayne printing co., fort wayne. (ESRI) environmental systems research institute, inc. 2005. ArcGIS Desktop Help, Release 9.1.Environmental Systems Research Institute, Inc., Redlands,
Farrer, J. L. (1995). Trees of the northern united states and canada. iowa state university press, ames. Federal Emergency Management Agency 2006 (FEMA).FEMA Flood Zones,
Foley, C. J. (2008).
Hanks, L. M. (1996). Body size influences mating success of the eucalyptus longhorned borer (coleoptera: Cerambycidae). Journal of Insect Behavior, 9(3), 369-382. doi:10.1007/BF02214016
Hanks, L. M. (1999). Influence of the larval host plant on reproductive strategies of cerambycid beetles doi:10.1146/annurev.ento.44.1.483
Hanks, L. M., Millar, J. C., & Paine, T. D. (1995). Biological constraints on host-range expansion by the wood-boring beetle phoracantha semipunctata (coleoptera: Cerambycidae). Annals of the Entomological Society of America, 88(2), 183-188. doi:10.1093/aesa/88.2.183
Hanks, L. M., Millar, J. G., & Paine, T. D. (1998). Dispersal of the eucalyptus longhorned borer (coleoptera: Cerambycidae) in urban landscapes. Environmental Entomology, 27(6), 1418-1424. doi:10.1093/ee/27.6.1418
Hanski, I. (1994). A practical model of metapopulation dynamics. Journal of Animal Ecology, 63(1), 151-162. doi:10.2307/5591
Hanula, J. L. (1993). Relationship of wood-feeding insects and coarse woody debris. in: J.W. McMinn & J.D.A. crossley (eds.): Workshop on the coarse woody debris in southern forests: Effects on biodiversity. USDA Forest Service, Athens,
Holland, J. D., Bert, D. G., & Fahrig, L. (2004). Determining the spatial scale of species' response to habitat. Bioscience, 54(3), 227-233. doi:10.1641/0006-3568(2004)054[0227:DTSSOS]2.0.CO;2
Hunter, M. D. (2002). Landscape structure, habitat fragmentation, and the ecology of insects. Agricultural and Forest Entomology, 4(3), 159-166. doi:10.1046/j.1461-9563.2002.00152.x
Hurvich, C. M., & Tsai, C. L. (1989). Regression and time series model selection in small samples. Biometrika, 76(2), 297-307. doi:10.1093/biomet/76.2.297
Jia, G. J., Burke, I. C., Goetz, A. F. H., Kaufmann, M. R., & Kindel, B. C. (2006). Assessing spatial patterns of forest fuel using AVIRIS data. Remote Sensing of Environment, 102(3-4), 318-327. doi:10.1016/j.rse.2006.02.025
Jopp, F., & Reuter, H. (2005). Dispersal of carabid beetles - emergence of distribution patterns. Ecological Modelling, 186(4), 389-405. doi:10.1016/j.ecolmodel.2005.02.009
Knull, J. N. (1946). The long-horned beetles of ohio (coleoptera: Cerambycidae). Bulletin of the Ohio Biological Survey, 39(4), 133-354.
Kotinsky, J. (1921).
Lacey, E. S., Ginzel, M. D., Millar, J. G., & Hanks, L. M. (2004). Male-produced aggregation pheromone of the cerambycid beetle neoclytus acuminatus acuminatus. Journal of Chemical Ecology, 30(8), 1493-1507. doi:10.1023/B:JOEC.0000042064.25363.42
Linsley, E. G. (1959). Ecology of cerambycidae. Annual Review of Entomology, 4, 99-138.
Linsley, E. G., & Chemsak, J. A. (1997).
Mattson, W., Vanhanen, H., Veteli, T., Sivonen, S., & Niemelä, P. (2007). Few immigrant phytophagous insects on woody plants in europe: Legacy of the european crucible? Biological Invasions, 9(8), 957-974. doi:10.1007/s10530-007-9096-y
Mazerolle, M. J. (2004).
Merriam, G. (1984). Connectivity: A fundamental ecological characteristic of landscape pattern. Methodology in Landscape Ecological Research and Planning, , 5-15.
Ray, N., Lehmann, A., & Joly, P. (2002). Modeling spatial distribution of amphibian populations: A GIS approach based on habitat matrix permeability. Biodiversity and Conservation, 11(12), 2143-2165. doi:10.1023/A:1021390527698
Roland, J., & Taylor, P. D. (1997). Insect parasitoid species respond to forest structure at different spatial scales. Nature, 386(6626), 710-713. doi:10.1038/386710a0
Roques, A. (2007).
Singleton, P. H., Gaines, W. L., & Lehmkuhl, J. F. (2004). Landscape permeability for grizzly bear movements in washington and southwestern british columbia. Ursus, 15(1), 90-103. doi:10.2192/1537-6176(2004)015<0090:LPFGBM>2.0.CO;2
Solomon, J. D. (1995).
Sork, V. L. (1983). Distribution of pignut hickory ( carya glabra) along a forest to edge transect, and factors affecting seedling recruitment. Bulletin - Torrey Botanical Club, 110(4), 494-506. doi:10.2307/2996284
Steffan-Dewenter, I., Münzenberg, U., Bürger, C., Thies, C., & Tscharntke, T. (2002). Scale-dependent effects of landscape context on three pollinator guilds. Ecology, 83(5), 1421-1432. doi:10.1890/0012-9658(2002)083[1421:SDEOLC]2.0.CO;2
Stevens, V. M., Verkenne, C., Vandewoestijne, S., Wesselingh, R. A., & Baguette, M. (2006). Gene flow and functional connectivity in the natterjack toad. Molecular Ecology, 15(9), 2333-2344. doi:10.1111/j.1365-294X.2006.02936.x
Sutcliffe, O. L., Bakkestuen, V., Fry, G., & Stabbetorp, O. E. (2003). Modelling the benefits of farmland restoration: Methodology and application to butterfly movement. Landscape and Urban Planning, 63(1), 15-31. doi:10.1016/S0169-2046(02)00153-6
Swihart, R. K., Goheen, J. R., Schnelker, S. A., & Rizkalla, C. E. (2007). Testing the generality of patch and landscape-level predictors of tree squirrel occurrence at a regional scale. Journal of Mammalogy, 88(3), 564-572. doi:10.1644/06-MAMM-A-275R.1
Taylor, P. D., Fahrig, L., Henein, K., & Merriam, G. (1993). Connectivity is a vital element of landscape structure. Oikos, 68(3), 571-573. doi:10.2307/3544927
Tirmenstein, D. A. (1991).
Verbeylen, G., De Bruyn, L., Adriaensen, F., & Matthysen, E. (2003). Does matrix resistance influence red squirrel (sciurus vulgaris L. 1758) distribution in an urban landscape? Landscape Ecology, 18(8), 791-805. doi:10.1023/B:LAND.0000014492.50765.05
Vogelmann, J. E., Howard, S. M., Yang, L., Larson, C. R., Wylie, B. K., & Van Driel, N. (2001).
Wagenmakers, E. -., & Farrell, S. (2004). AIC model selection using akaike weights. Psychonomic Bulletin and Review, 11(1), 192-196. doi:10.3758/BF03206482
Waters, D. J. (1981). Life history of neoclytus acuminatus with notes on other cerambycids associated with dead or dying deciduous trees. MS Thesis.Auburn University, Auburn,
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2019 Carolyn J. Foley, Jeffrey D. Holland

This work is licensed under a Creative Commons Attribution 4.0 International License.